Page 1569 - MISUMI Thailand Economy Series
P. 1569
[Technical Data]
SI (International System of Units) Excerpted from GB 3100—1993
1. International System of Units(SI)and Usage. 1-3. Multiples of 10 of SI Units
1-1. Scope of Application This standard specifies how to use the International System of Units(SI) and other international unitary systems, as well as (1) Prefix The multiples and the names and symbols of prefixes to express integer multiples of 10 of SI Units are shown in Table 4.
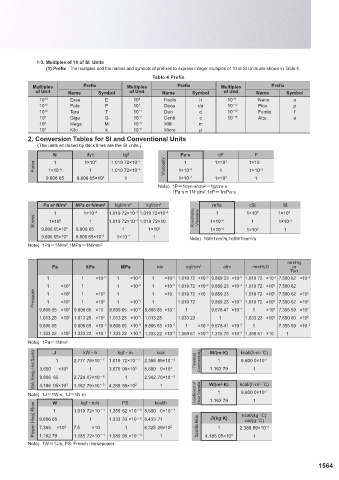
units used in correlation with units from international systems, and other units which may be used. Table 4 Prefix.
1-2. Terms and Definitions Terminology used in this specification and definitions thereof are as follows. Multiples Prefix Multiples Prefix Multiples Prefix
(1) International System of Units(SI) Coherent system of units adopted and recommended by the International Committee on Weights and Measures. of Unit Name Symbol of Unit Name Symbol of Unit Name Symbol
It contains base units and supplementary units, units derived from them and their integral exponents to the 10th power. 10 18 Exsa E 10 2 Hecto h 10 −9 Nano n
(2) SI Unit Generic term used to describe base units, supplementary units or derived units of the International System of Units(SI). 10 15 Peta P 10 1 Deca da 10 −12 Pico p
(3) Base Unit Those units are given in Table 1. 10 12 Tera T 10 −1 Deci d 10 −15 Femto f
(4) Supplementary Units Those supplementary units are given in Table 2. 10 9 Giga G 10 −2 Centi c 10 −18 Atto a
10 6 Mega M 10 −3 Milli m
10 3 Kilo k 10 −6 Micro μ
Base Quantity Unit Symbol Definition 2. Conversion Tables for SI and Conventional Units
A meter is the length of the path traveled by light in a vacuum during a time interval (The units enclosed by thick lines are the SI units.)
Length Meter m of of a second. N dyn kgf Pa·s cP P
1
299 792 458 5 −1 3
1
1
Mass Kilogram kg A kilogram is a unit of mass (neither weight nor force), it is equal to the mass of the Force 1×10 −5 1×10 1.019 72×10 −6 Viscosity 1×10 −3 1×10 1×10 −2
international prototype of the kilogram.
1
1
1.019 72×10
1×10
Time Second s Second is the duration of 9,192,631,770 periods of the radiation corresponding to the 9.806 65 9.806 65×10 5 1 1×10 −1 1×10 2 1
transition between the two hyperfine levels of the ground state of the cesium-133 atom
An ampere is that constant current which, if maintained in two straight parallel conductors Note) 1P=1dyn·s/cm 2 =1g/cm·s
Current Ampere A of infinite length, of negligible circular cross-section, and placed 1 meter apart in a vacuum, 1Pa·s=1N·s/m 2 ,1cP=1mPa·s
would produce between these conductors a force equal to 2×10 Newton per meter of length.
−7
1
Thermodynamic Kelvin, a unit of thermodynamic temperature, is the fraction of the Pa or N/m 2 MPa or N/mm 2 kgf/mm 2 kgf/cm 2 m /s cSt St
2
Temperature Kelvin K thermodynamic temperature of the triple point of water. 273.16 1 1×10 −6 1.019 72×10 −7 1.019 72×10 −5 1 1×10 6 1×10 4
Amount of Mole mol A mole is the amount of substance of a system that contains as many elementary 1×10 6 1 1.019 72×10 −1 1.019 72×10 Kinematic Viscosity 1×10 −6 1 1×10 −2
particles(1) or aggregation of elementary particles as there are atoms in 0.012 kilogram
Substance of carbon 12 and when the mole is used, the elementary particles must be specified. Stress 6 2 −4 2
A candela is the luminous intensity, in a given direction, of a source that emits 9.806 65×10 9.806 65 1 1×10 1×10 1×10 1
Luminance Candela cd monochromatic radiation of frequency 540×10 hertz and that has a radiant 9.806 65×10 4 9.806 65×10 −2 1×10 −2 1 Note) 1St=1cm /s,1cSt=1mm /s
2
2
12
Intensity intensity in that direction of watt per steradian. Note) 1Pa=1N/m 2 ,1MPa=1N/mm 2
1
683
Note( ) The elementary particles here must be atoms, molecules, ions, electrons or other particles.
1
mmHg
Pa kPa MPa bar kgf/cm 2 atm mmH2O or
Torr
Base Quantity Unit Symbol Definition 1 1 ×10 −3 1 ×10 −6 1 ×10 −5 1.019 72 ×10 −5 9.869 23 ×10 −6 1.019 72 ×10 −1 7.500 62 ×10 −3
A radian is the plane angle between two radii of a circle that cuts off an arc on the
Plane Angle Radian rad 1 ×10 3 1 1 ×10 −3 1 ×10 −2 1.019 72 ×10 −2 9.869 23 ×10 −3 1.019 72 ×10 2 7.500 62
circumference equal in length to the radius.
Solid Angle Steradian sr A steradian is the solid angle which, having its vertex in the center of a sphere, cuts off an area of the Pressure 1 1 ×10 6 5 1 1 ×10 3 2 1 1 ×10 −1 1 1 ×10 1.019 72 ×10 9.869 23 −1 1.019 72 ×10 5 4 7.500 62 ×10 3 2
surface of the sphere equal to that of a square with sides equal in length to the radius of the sphere.
1.019 72
7.500 62 ×10
×10
9.869 23 ×10
1.019 72 ×10
×10
9.806 65 ×10 4 9.806 65 ×10 9.806 65 ×10 −2 9.806 65 ×10 −1 1 9.678 41 ×10 −1 1 ×10 4 7.355 59 ×10 2
1.013 25 ×10 5 1.013 25 ×10 2 1.013 25 ×10 −1 1.013 25 1.033 23 1 1.033 23 ×10 4 7.600 00 ×10 2
Table 3. SI Derived Units with Special Names and Symbols
9.806 65 9.806 65 ×10 −3 9.806 65 ×10 −6 9.806 65 ×10 −5 1 ×10 −4 9.678 41 ×10 −5 1 7.355 59 ×10 −2
Base Quantity Expression in Terms of Base 1.333 22 ×10 2 1.333 22 ×10 −1 1.333 22 ×10 −4 1.333 22 ×10 −3 1.359 51 ×10 −3 1.315 79 ×10 −3 1.359 51 ×10 1
Base Quantity Units or Supplementary
Units, Supplementary Units
Name Symbol or Other SI Units Note) 1Pa=1N/m 2
Frequency Hertz Hz 1 Hz =1 s −1 J kWCh kgfCm kcal W/(m·K) kcal/(h·m·˚C)
Force Newton N 1 N =1 kg·m/s 2 1 2.777 78×10 -7 1.019 72×10 -1 2.388 89×10 -4 Thermal Conductivity 1 8.600 0×10 −1
Pressure, Stress Pascal Pa 1 Pa =1 N/m 2 3.600 ×10 6 1 3.670 98×10 5 8.600 0×10 2 1.162 79 1
Energy, Work, Heat Quantity Joule J 1 J =1 N·m Work, Energy, Heat Quantity -6 -3
Work Rate, Process Rate, Power, Electric Power Watt W 1 W =1 J/s 9.806 65 2.724 07×10 1 2.342 70×10
2
2
Electric Charge, Quantity of Electricity Coulomb C 1 C =1 A·s 4.186 05×10 3 1.162 79×10 -3 4.268 58×10 2 1 W/(m ·K) kcal/(h·m ·˚C)
Electric Potential, Potential Difference, Voltage, Electromotive Force Volts V 1 V =1 J/C Note) 1J=1W·s, 1J=1N·m Coefficient of Heat Transfer 1 8.600 0×10 −1
Electrostatic Capacity, Capacitance Farad F 1 F =1 C/V 1.162 79 1
Electric Resistance Ohm Ω 1 Ω =1 V/A W kgfCm/s PS kcal/h
Conductance Siemens S 1 S =1 Ω −1 1 1.019 72×10 -1 1.359 62 ×10 -3 8.600 0×10 -1
Magnetic Flux Weber Wb 1 Wb =1 V·s Power Heat Flow 9.806 65 1 1.333 33 ×10 -2 8.433 71 J/(kg·K) kcal/(kg·˚C)
cal/(g·˚C)
Magnetic Flux Density Tesla T 1 T =1 Wb/m 2 7.355 ×10 2 7.5 ×10 1 6.325 29×10 2 Specific Heat 1 2.388 89×10 −4
Inductance Henry H 1 H =1 Wb/A -1 -3
Celsius Temperature Degree Celsius ˚C 1 t˚C =(t+273.15)k 1.162 79 1.185 72×10 1.580 95 ×10 1 4.186 05×10 3 1
Luminous Flux Lumen lm 1 lm =1 cd·sr Note) 1W=1J/s, PS: French Horsepower
Illuminance Lux lx 1 lx =1 lm/m 2
Radioactivity Becquerel Bq 1 Bq =1 s −1
1563 Absorbed Dose Gray Gy 1 Gy =1 J/kg 1564
Dose Equivalent Sievert Sv 1 Sv =1 J/kg
KJTIV JOEC
KJTIV JOEC